
The hardware and bandwidth for this mirror is donated by dogado GmbH, the Webhosting and Full Service-Cloud Provider. Check out our Wordpress Tutorial.
If you wish to report a bug, or if you are interested in having us mirror your free-software or open-source project, please feel free to contact us at mirror[@]dogado.de.
Song Lab 2021-10-31
Notice:
We add new features in tvt.eSIR(). Now the new
function tvt.eSIR2() adds time_unit which will
allow users to change their time unit from 1 day to any arbitrary
numbers of days.
Since version 0.3.1, we added the US data. The most recent update was on June 25th, 2021. We used data summarized from JHU CSSE GitHub and 1Point3Acres. Only 41 states have complete recovery data. We will update the data every Thursday or Friday evening. Using following codes for the data:
data("confirmed") # From JHU CSSE
data("death") # From JHU CSSE
data("recovered") # partly from 1Point3Acres
data("USA_state_N") #population in each staterjags.
Their values substantially larger than 1 imply a failure of convergence
of the MCMC chains. Save it in a .txt file by setting
save_files = TRUE or retrieve it via following R code
(assuming that res is object returned from
tvt.eSIR or qh.eSIR):res$gelman_diag_listChinese version:中文
The outbreak of novel Corona Virus disease (a.k.a. COVID-19), originated in Wuhan, the capital of Hubei Province spreads quickly and affects many cities in China as well as many countries in the world. The Chinese government has enforced very stringent quarantine and inspection to prevent the worsening spread of COVID-19. Although various forms of forecast on the turning points of this epidemic within and outside Hubei Province have been published in the media, none of the prediction models has explicitly accounted for the time-varying quarantine protocols. We extended the classical SIR model for infectious disease by incorporating forms of medical isolation (in-home quarantine and hospitalization) in the underlying infectious disease dynamic system. Using the state-space model for both daily infected and hospitalized incidences and MCMC algorithms, we assess the effectiveness of quarantine protocols for confining COVID-19 spread in both Hubei Province and the other regions of China. Both predicted turning points and their credible bands may be obtained from the extended SIR under a given quarantine protocol. R software packages are also made publicly available for interested users.
The standard SIR model has three components: susceptible, infected,
and removed (including the recovery and dead). In the following
sections, we will introduce the other extended state-space SIR models
and their implementation in the package. The results provided
below are based on relatively short chains. According to our
experience, this setting (M=5e3 and
nburnin=2e3) should provide acceptable results in terms of
the trend and turning points estimation, the estimation of parameters
and their credible intervals might not be accurate. Hence, if possible,
we would recommend using M=5e5 and nburnin=2e5
to obtain stable MCMC chains via rjags.





To install and use this R package from Github, you will need to first
install the R package devtools. Please uncomment the codes
to install them. eSIR depends on three other packages,
rjags (an interface to the JAGS library),
chron and gtools, which could be installed
with eSIR if not yet.
An error may occur if you have not yet installed JAGS-4.x.y.exe (for any x >= 0, y >=0). Windows users may download and install JAGS from here. Mac users may follow steps at casallas/8411082.
# install.packages("devtools")
# library(devtools)
# install_github("lilywang1988/eSIR")
library(eSIR) Our data are collected daily from dxy dot com. Alternatively, we notice some convenient access to COVID-19 data from GuangchuangYu/nCov2019.
For data outside China, we use JHU CSSE GitHub data. Another package coronavirus has its GitHub version udpated daily, which is also quite useful.
# Data of COVID-19 can be found in the following R packages:
# install_github("GuangchuangYu/nCov2019")
#library(nCov2019)
# install_github("qingyuanzhao/2019-nCov-Data")
#library(2019-nCov-Data) In Ubuntu (18.04) Linux, please first update R to a version >=
3.6. You may need to install jags package as well by
sudo apt-get install jags before install devtools by
install.packages("devtools"). This
stackoverflow question perfectly solved the issue I encountered when
installing devtools on ubuntu. You may follow this gist
to resolve other possible issues when installing eSIR on
the recent version of Ubuntu.
tvt.eSIR(): a SIR model with a time-varying
transmission rateBy introducing a time-dependent [(t)] function that modifies the transmission rate [], we can depict a series of time-varying changes caused by either external variations like government policies, protective measures and environment changes, or internal variations like mutations and evolutions of the pathogen.
The function can be either stepwise or exponential:


set.seed(20192020)
library(eSIR)
# Hubei province data Jan13 -> Feb 11
# Cumulative number of infected cases
NI_complete <- c(
41, 41, 41, 45, 62, 131, 200, 270, 375, 444, 549, 729,
1052, 1423, 2714, 3554, 4903, 5806, 7153, 9074, 11177,
13522,16678,19665,22112,24953,27100,29631,31728,33366
)
# Cumulative number of removed cases
RI_complete <- c(
1, 1, 7, 10, 14, 20, 25, 31, 34, 45, 55, 71, 94,
121, 152, 213, 252, 345, 417, 561, 650, 811,
1017, 1261, 1485, 1917, 2260, 2725, 3284, 3754
)
N <- 58.5e6
R <- RI_complete / N
Y <- NI_complete / N - R #Jan13->Feb 11
### Step function of pi(t)
change_time <- c("01/23/2020", "02/04/2020", "02/08/2020")
pi0 <- c(1.0, 0.9, 0.5, 0.1)
res.step <- tvt.eSIR(Y,
R,
begin_str = "01/13/2020",
T_fin = 200,
pi0 = pi0,
change_time = change_time,
dic = FALSE,
casename = "Hubei_step",
save_files = FALSE,
save_mcmc = FALSE,
save_plot_data = TRUE, # can plot them
M = 5e3,
nburnin = 2e3 # additional to M
)
#> The follow-up is from 01/13/20 to 07/30/20 and the last observed date is 02/11/20.
#> Running for step-function pi(t)
#> Compiling model graph
#> Resolving undeclared variables
#> Allocating nodes
#> Graph information:
#> Observed stochastic nodes: 60
#> Unobserved stochastic nodes: 36
#> Total graph size: 1875
#>
#> Initializing model
res.step$plot_infection
res.step$plot_removed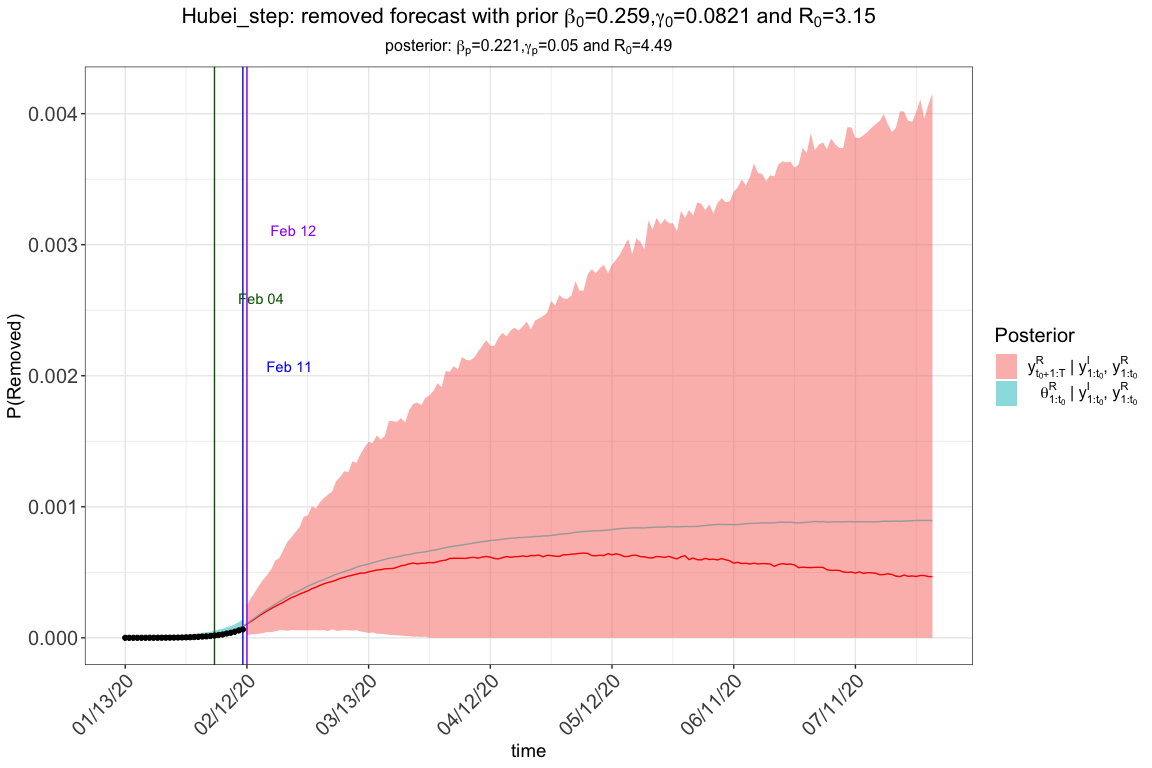
res.step$spaghetti_plot
# If we change the time unit to 5 days
# Cumulative number of infected cases
NI_complete2 <- NI_complete[seq(1, length(NI_complete), 5)]
# Cumulative number of removed cases
RI_complete2 <- RI_complete[seq(1, length(RI_complete), 5)]
N2 <- 58.5e6
R2 <- RI_complete2 / N2
Y2 <- NI_complete2 / N2 - R2 #Jan13->Feb 11
change_time2 <- c("01/23/2020", "02/04/2020", "02/08/2020")
pi02 <- c(1.0, 0.9, 0.5, 0.1)
res.step2 <- tvt.eSIR2(Y2,
R2,
begin_str = "01/13/2020",
T_fin = 40,
pi0 = pi02,
change_time = change_time,
dic = FALSE,
casename = "Hubei_step2",
save_files = FALSE,
save_mcmc = FALSE,
save_plot_data = TRUE,
M = 5e3,
nburnin = 2e3,
time_unit = 5 #new feature!
)
#> The follow-up is from 01/13/20 to 07/26/20 and the last observed date is 02/07/20.
#> Running for step-function pi(t)
#> Compiling model graph
#> Resolving undeclared variables
#> Allocating nodes
#> Graph information:
#> Observed stochastic nodes: 12
#> Unobserved stochastic nodes: 12
#> Total graph size: 395
#>
#> Initializing model
res.step2$plot_infection
res.step2$plot_removed
res.step2$spaghetti_plot
res.step2$dic_val
#> NULL
#res.step$gelman_diag_list
### continuous exponential function of pi(t)
res.exp <- tvt.eSIR(Y,
R,
begin_str = "01/13/2020",
death_in_R = 0.4,
T_fin = 200,
exponential = TRUE,
dic = FALSE,
lambda0 = 0.05,
casename = "Hubei_exp",
save_files = FALSE,
save_mcmc = FALSE,
save_plot_data = TRUE,
M = 5e3,
nburnin = 2e3)
#> The follow-up is from 01/13/20 to 07/30/20 and the last observed date is 02/11/20.
#> Running for exponential-function pi(t)
#> Compiling model graph
#> Resolving undeclared variables
#> Allocating nodes
#> Graph information:
#> Observed stochastic nodes: 60
#> Unobserved stochastic nodes: 36
#> Total graph size: 1875
#>
#> Initializing model
res.exp$plot_infection
res.exp$spaghetti_plot
#res.exp$plot_removed
# Again, if we change our time unit to 5 days
res.exp2 <- tvt.eSIR2(Y2,
R2,
begin_str = "01/13/2020",
death_in_R = 0.4,
T_fin = 200,
exponential = TRUE,
dic=FALSE,
lambda0 = 0.05,
casename = "Hubei_exp2",
save_files = FALSE,
save_mcmc = FALSE,
save_plot_data = TRUE,
M = 5e3,
nburnin = 2e3,
time_unit = 5)
#> The follow-up is from 01/13/20 to 10/04/22 and the last observed date is 02/07/20.
#> Running for exponential-function pi(t)
#> Compiling model graph
#> Resolving undeclared variables
#> Allocating nodes
#> Graph information:
#> Observed stochastic nodes: 12
#> Unobserved stochastic nodes: 12
#> Total graph size: 555
#>
#> Initializing model
res.exp2$plot_infection
res.exp2$spaghetti_plot
### without pi(t), the standard state-space SIR model without intervention
res.nopi <- tvt.eSIR(Y,
R,
begin_str = "01/13/2020",
death_in_R = 0.4,
T_fin = 200,
casename = "Hubei_nopi",
save_files = F,
save_plot_data = F,
M=5e3,nburnin = 2e3)
#> The follow-up is from 01/13/20 to 07/30/20 and the last observed date is 02/11/20.
#> Running without pi(t)
#> Compiling model graph
#> Resolving undeclared variables
#> Allocating nodes
#> Graph information:
#> Observed stochastic nodes: 60
#> Unobserved stochastic nodes: 36
#> Total graph size: 1875
#>
#> Initializing model
res.nopi$plot_infection
res.nopi$spaghetti_plot
#res.nopi$plot_removedqh.eSIR(): SIR with time-varying quarantine, which
follows a Dirac Delta functionBy introducing a vector of phi and its corresponding
changing points change_time, we introduced a quarantine
process that is dependent on a dirac delta function (_t). In other
words, only at time points defined by change_time, we have
certain porportions of the at-risk (susceptible) subjects moved to the
quarantine stage. The difference of this model than the previous
time-varying transmission one is that we do not allow the tranmission
rate to change, but only let the proportion of susceptible subjects
decrease. 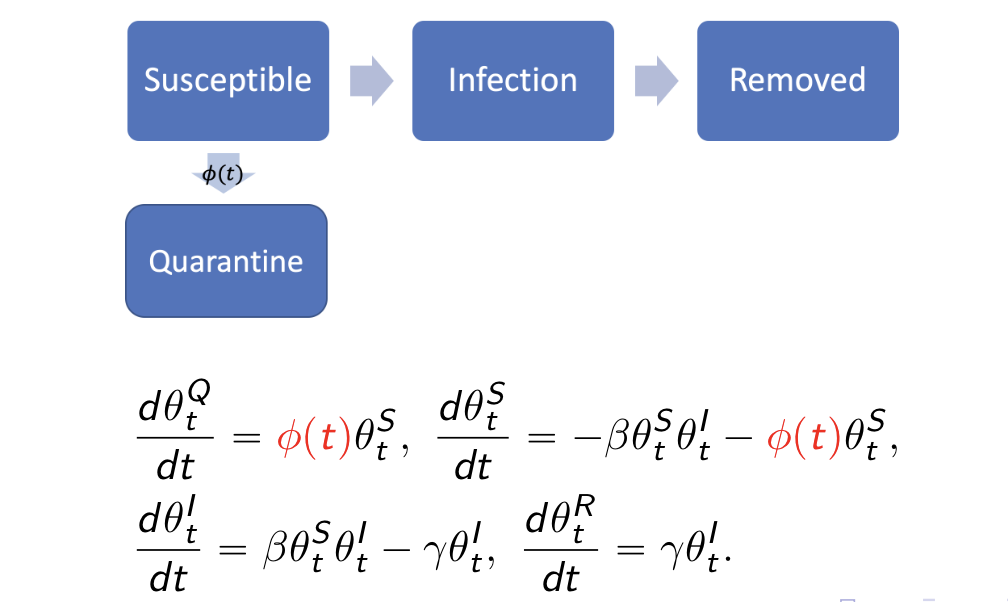

set.seed(20192020)
# Cumulative number of infected cases
NI_complete <- c(
41, 41, 41, 45, 62, 131, 200, 270, 375, 444,
549, 729, 1052, 1423, 2714, 3554, 4903, 5806,
7153, 9074, 11177, 13522, 16678, 19665, 22112,
24953, 27100, 29631, 31728, 33366)
# Cumulative number of removed cases
RI_complete <- c(
1, 1, 7, 10, 14, 20, 25, 31, 34, 45, 55,
71, 94, 121, 152, 213, 252, 345, 417, 561,
650, 811, 1017, 1261, 1485, 1917, 2260,
2725, 3284, 3754)
N <- 58.5e6
R <- RI_complete / N
Y <- NI_complete / N - R #Jan13->Feb 11
change_time <- c("01/23/2020", "02/04/2020", "02/08/2020")
phi0 <- c(0.1, 0.4, 0.4)
res.q <- qh.eSIR(Y,
R,
begin_str = "01/13/2020",
phi0 = phi0,
change_time = change_time,
casename = "Hubei_q",
save_files = TRUE,
save_mcmc = FALSE,
save_plot_data = FALSE,
M = 5e3,
nburnin = 2e3)
#> The follow-up is from 01/13/20 to 07/30/20 and the last observed date is 02/11/20.
#> Running for qh.eSIR
#> Compiling model graph
#> Resolving undeclared variables
#> Allocating nodes
#> Graph information:
#> Observed stochastic nodes: 60
#> Unobserved stochastic nodes: 36
#> Total graph size: 2736
#>
#> Initializing model
res.q$plot_infection
You will obtain the following plot in addition to the traceplots and
summary table if you set save_file=T in
qh.eSIR. The blue vertical line denotes the beginning date,
and the other three gray lines denote the three change points.

eSAIR: SIR with time-varying subset of population
immunized with antibody positivityTo address the under-reporting issue associated with the available public databases and to build the self-immunization into the infection dynamics, we then further extend the previous eSIR model to an eSAIR model by adding an antibody (A) compartment. As is shown in the bottom thread of the following figure, this A compartment accounts for the probability of being self-immunized with antibodies to COVID-19, denoted by (_t^A); see equation as follows too, where ((t)) is a function describing the proportion of people moving from the susceptible compartment to the antibody compartment over time. Compartment A helps circumvent limitations of data collection, especially embracing individuals who were infected but self-cured at home with no confirmation by viral RT-PCR diagnostic tests. This new eSAIR model characterizes the underlying population-level dynamics of the pandemic. The following system of ordinary differential equations defines collectively the continuous-time dynamics for the eSAIR model, which governs the law of movements among four compartments of Susceptible, Self-immunized, Infected and Removed.


In the example below, we implemented this function onto New York state data assuming that by April 29, 20% of the population have been self-immunized with antibody positivity.
# Cumulative number of infected cases
NI_complete <- c(
1, 2, 11, 23, 31, 76, 106, 142, 150, 220, 327,
421, 613, 615, 967, 1578, 3038, 5704, 8403,
11727, 15800, 20884, 25681, 30841, 37877,
44876, 52410, 59648, 66663, 75833, 83948,
92506, 102987, 113833, 123160, 131815,
139875, 151061, 161779, 172348, 181026,
189033, 195749, 203020, 214454, 223691,
230597, 237474, 243382, 248416, 253519,
258222, 263460, 271590, 282143, 288045,
291996, 295106, 299691, 304372, 308314)
# Cumulative number of removed cases
RI_complete <- c(
0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 1, 2,
20, 26, 38, 52, 200, 311, 468, 662,
893, 1144, 1547, 2144, 2931, 3900,
5133, 6201, 7657, 9483, 11536,
13915, 16276, 18781, 21110, 23424,
26469, 29784, 32899, 35785, 37730,
39207, 40703, 42164, 43488, 44723,
45887, 47473, 47686, 48769, 49572,
50221, 52161, 52917, 54115, 54613,
55473, 55816, 56809, 57265, 58525)
N <- 8.399e6
R <- RI_complete / N
Y <- NI_complete / N - R
change_time <- c("04/29/2020")
alpha0 <- c(0.2) # 20% of the susceptible population were found immunized
res.antibody <- eSAIR(Y,
R,
begin_str = "03/01/2020",
alpha0 = alpha0,
change_time = change_time,
casename = "New_York_antibody",
save_files = F,
save_mcmc = F,
M=5e2,
nburnin = 2e2)
#> The follow-up is from 03/01/20 to 09/16/20 and the last observed date is 04/30/20.
#> Running for eSAIR
#> Compiling model graph
#> Resolving undeclared variables
#> Allocating nodes
#> Graph information:
#> Observed stochastic nodes: 122
#> Unobserved stochastic nodes: 67
#> Total graph size: 5123
#>
#> Initializing model
#>
#> NOTE: Stopping adaptation
res.antibody$plot_infection
To save all the plots (including trace plots) and summary tables,
please set save_files=T, and if possible, provide a
location by setting file_add="YOUR/FAVORITE/FOLDER".
Otherwise, the traceplots and other intermediate plots will not be
saved, but you can still retrieve the forecast plots and summary table
based on the return list, e.g., using
res.step$forecast_infection and
res.step$out_table. Moreover, if you are interested in
plotting the figures on your own, you may set save_mcmc=T
so that all the MCMC draws will be saved in a .RData file
too.
For details, please explore our package directly. We have
.rd files established, please use
help(tvt.eSIR) or ?qh.eSIR to find them.
This package is created and maintained by Lili Wang, contributed by Fei Wang, Lu Tang, and Paul Egeler. We also thank Kangping Yang for helping us collect the recovery data from the web page.
Wang, L., Zhou, Y., He, J., Zhu, B., Wang, F., Tang, L., … & Song, P. X. (2020). An epidemiological forecast model and software assessing interventions on the COVID-19 epidemic in China. Journal of Data Science, 18(3), 409-432. link
Osthus, D., Hickmann, K. S., Caragea, P. C., Higdon, D., & Del Valle, S. Y. (2017). Forecasting seasonal influenza with a state-space SIR model. The annals of applied statistics, 11(1), 202.
Gelman, A., & Rubin, D. B. (1992). Inference from iterative simulation using multiple sequences. Statistical science, 7(4), 457-472.
This work is licensed under a Creative Commons Attribution 4.0 International License.
These binaries (installable software) and packages are in development.
They may not be fully stable and should be used with caution. We make no claims about them.
Health stats visible at Monitor.